STPM Semester 1 Chemistry Experiment 3 : Volumetric Analysis - Purity and stoichiometry
Hey Moons! Hope you can benefit from this!
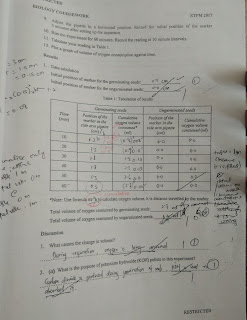
Below here are the question and basic report.
While here are my report. Pardon the terrible writing. The calculation part are the important ones.
Here I'll insert some of the pictures I took.
These are the three solutions + with KA4 which is solid sodium hydrogencarbonate.

Right are before KA3 is added. Left are after KA3 is added. Notice that it became slightly darker.

This is after all three solution were added with four drops of starch (minimum is 3 drops). Supposedly it turns dark blue, it did when I drop in the starch but after that it just became a darker shade of brownish grey so I don't know what happened. Everyone got the same and all the chem teacher said normal sooooooo *shrugs*
This is after titration. Stop when the solution turns colourless and record the final volume.
So there you go. Always remember to make sure there are no bubbles in your burette before conducting the experiment and constantly shake the titration / conical flask throughout the entire experiment.
Byeeeeeee
Below here are the question and basic report.
While here are my report. Pardon the terrible writing. The calculation part are the important ones.
These are the three solutions + with KA4 which is solid sodium hydrogencarbonate.

Right are before KA3 is added. Left are after KA3 is added. Notice that it became slightly darker.

This is after all three solution were added with four drops of starch (minimum is 3 drops). Supposedly it turns dark blue, it did when I drop in the starch but after that it just became a darker shade of brownish grey so I don't know what happened. Everyone got the same and all the chem teacher said normal sooooooo *shrugs*
This is after titration. Stop when the solution turns colourless and record the final volume.
So there you go. Always remember to make sure there are no bubbles in your burette before conducting the experiment and constantly shake the titration / conical flask throughout the entire experiment.
Byeeeeeee













Thank u very muchhhh !!!!
ReplyDelete